4 Groundbreaking IgG4-RD Scientific Discoveries That You Need to Know About

IgG4-Related Disease (IgG4-RD) is a rare immune-mediated condition that can impact nearly every organ in the body. As researchers are still studying how IgG4-RD works, many new studies have emerged that examine treatments, diagnostic journeys, and outcomes for people living with IgG4-Rd.
In this post, we break down four of the most impactful and recent IgG4-RD lab science studies that provide key insight into this condition and potential treatment methods for healthcare providers and patients.
Why is AiArthritis raising awareness about IgG4-RD? Although IgG4-RD is not officially part of the AiArthritis disease umbrella, AiArthritis is raising awareness because it may end up being classified as autoimmune or autoinflammatory in the future. Additionally, as IgG4-RD is often confused with other conditions, many people with IgG4-RD may be misdiagnosed with an AiArthritis disease or face challenges getting a diagnosis.
Early Results from a Study Testing the Safety of Rilzabrutinib for IgG4-RD Patients
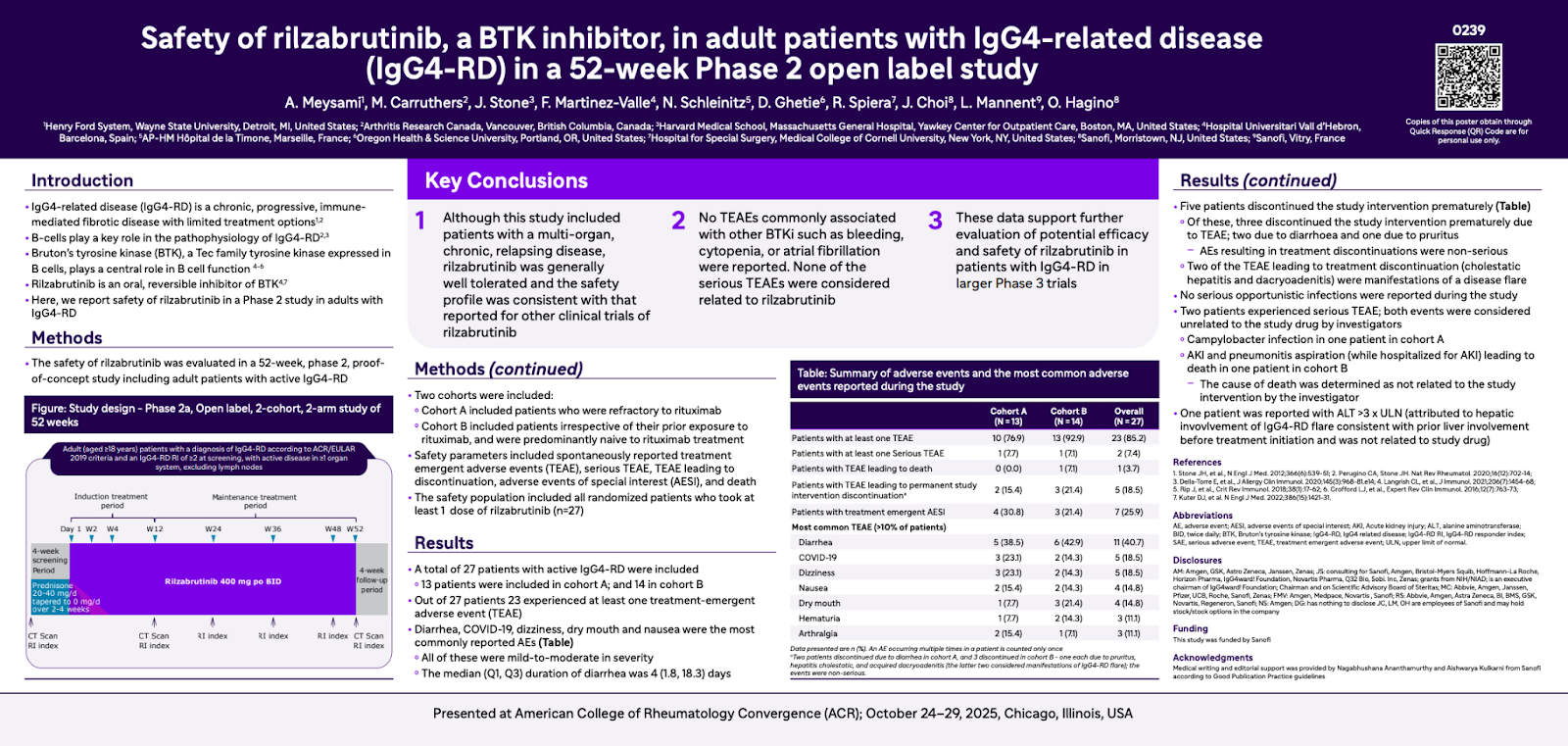
Safety of Rilzabrutinib, a BTK Inhibitor, in Adult Patients with IgG4-related disease (IgG4-RD) in a 52-week Phase 2 Open-label Study
Summary
This table shows how often different side effects (also called adverse events) happened among people who took rilzabrutinib in a research study. Overall, the treatment was generally well tolerated, meaning most people could continue taking it without serious issues.
Most side effects were mild to moderate, such as stomach problems or mild infections. Only two people (7%) had serious side effects, and there was one death reported (3.7%), which was considered related to a treatment-emergent adverse event (TEAE).
These findings suggest that rilzabrutinib may be a promising therapy with an acceptable safety profile, though more research is needed to confirm its safety and effectiveness in larger studies.
Questions Answered by this Poster
- What types of side effects occurred in people taking rilzabrutinib?
- How many participants experienced side effects, and how severe were they?
- Were any serious or life-threatening side effects seen?
- Are the side effects tolerable enough for future studies to continue?
Methods
- Participants were divided into two cohorts:
- Cohort A: 13 participants
- Cohort B: 14 participants
- Total: 27 participants
- Side effects were recorded and grouped by category, using international medical standards (MedDRA 27.1).
- Researchers tracked any side effects that started after participants began rilzabrutinib.
Results
- 85% of participants had at least one side effect.
- 7% experienced a serious side effect.
- 19% had to stop the treatment due to side effects.
- The most common side effects were:
- Gastrointestinal problems (59%) – such as nausea or diarrhea
- Infections (44%) – typically mild
- Skin issues (41%) – like rashes or irritation
- Nervous system symptoms (30%) – possibly headaches or fatigue
Conclusions
Most patients tolerated rilzabrutinib well, with few serious events and a manageable side effect profile. The study supports continuing research into rilzabrutinib as a potential therapy for autoimmune diseases such as IgG4-RD.
Key Data
- 59% reported gastrointestinal disorders
- 44% reported infections
- 41% reported skin disorders
- 30% reported nervous system disorders
Authors
A. Meysami (Amgen, AstraZeneca, GSK, Janssen, Zenas); M. Carruthers (AbbVie, Amgen, Janssen, Pfizer, Roche, Sanofi, UCB, Zenas); J. Stone (Acepodia, Amgen, argenx, Bristol-Myers Squibb, Novartis, Q32 Bio, Sanofi, Zenas); F. Martinez-Valle (Amgen); N. Schleinitz (Amgen); D. Ghetie (None); R. Spiera (AbbVie, Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Certa, Chemocentryx, Corbus, Cytori, Galderma, GSK, Horizon, Kadmon, Novartis, Principia, Regeneron, Roche-Genentech, Sanofi, Vera Therapeutic); J. Choi, L. Mannent, and O. Hagino (Sanofi).
Read the published abstract in ACR Meeting Abstracts
Major International Study (MITIGATE) Shows Promise as a Safe, Effective Long-Term Treatment
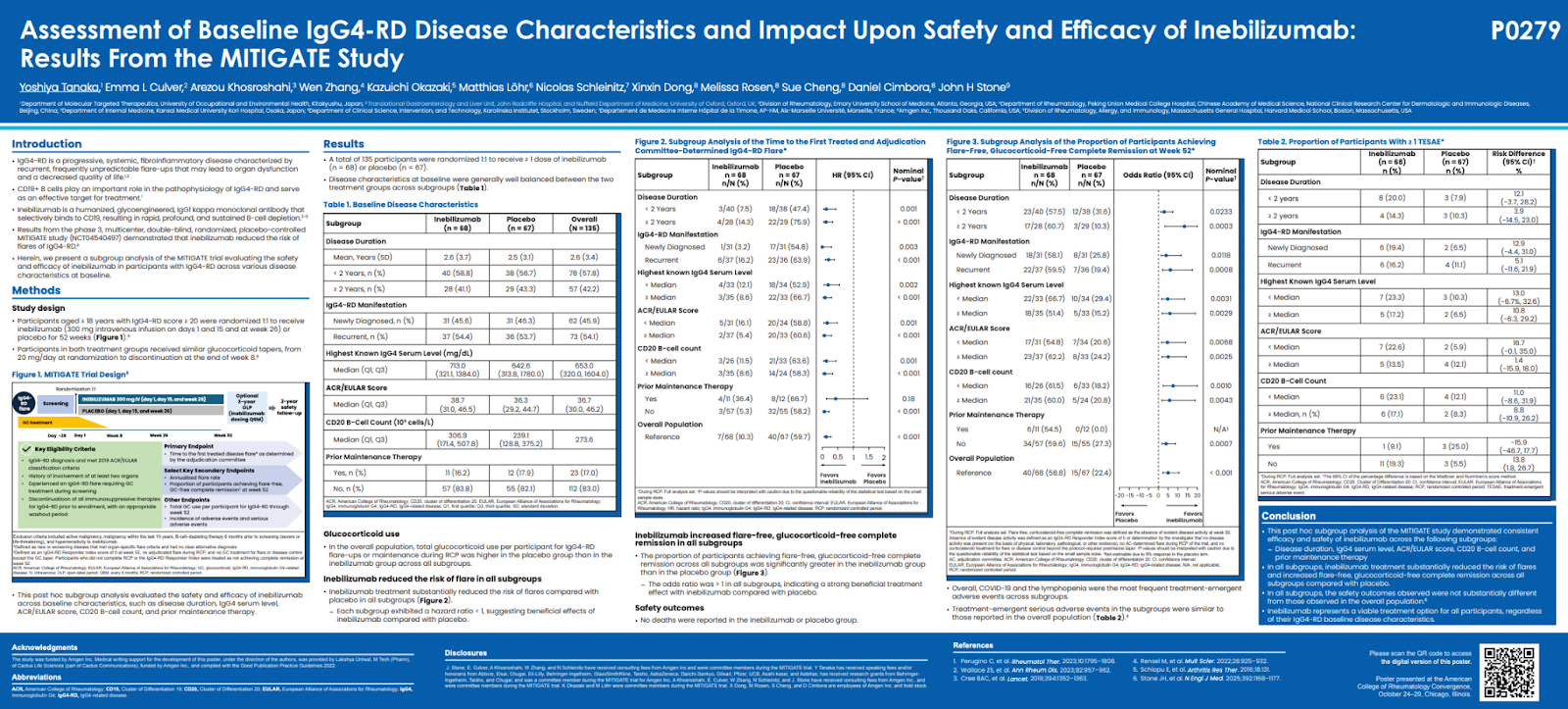
Assessment of Baseline IgG4-RD Disease Characteristics and Impact upon Safety and Efficacy of Inebilizumab: Results from the MITIGATE Study
Summary
The MITIGATE trial is a major international study evaluating a potential new treatment — inebilizumab — for IgG4-related disease (IgG4-RD), a chronic condition where the immune system causes inflammation and scarring in multiple organs.
Patients living with IgG4-RD often face recurrent flares, unpredictable organ involvement, and the long-term side effects of repeated steroid use. This study found that inebilizumab, a targeted therapy that removes certain overactive immune B cells, can reduce flares, prolong remission, and help patients stay off corticosteroids — regardless of how long they’ve had the disease, how high their IgG4 levels are, or how many organs are affected.
Importantly, the benefits were consistent across all patient subgroups and the treatment was generally well tolerated.
Questions Answered by this Poster
- Does inebilizumab work equally well in patients with different disease histories and lab profiles?
- Can inebilizumab reduce IgG4-RD flares and maintain remission without steroids?
- Is the treatment safe across all types of patients with IgG4-RD?
Methods
- Design: Phase 3, international, randomized, double-blind, placebo-controlled trial (MITIGATE; NCT04540497).
- Participants: 135 adults meeting ACR/EULAR classification criteria for IgG4-RD (score ≥20), all with multiorgan involvement and a recent flare requiring steroids.
- Groups: Inebilizumab (INEB, n=68) vs placebo (PBO, n=67).
- Primary Endpoint: Time to first adjudication committee (AC)-determined, investigator-treated IgG4-RD flare during the 52-week randomized controlled period (RCP).
- Key Secondary Endpoints:
- Flare-free, corticosteroid-free complete remission at Week 52.
- Annualized flare rate.
- Disease activity score (ACR/EULAR).
- Safety and tolerability.
- Subgroup Analysis: By disease duration (<2 vs ≥2 years), diagnosis type (new vs recurrent), serum IgG4 level, ACR/EULAR score, CD20 B-cell count, and prior maintenance therapy.
Results
- Flare Prevention: Inebilizumab reduced flare risk by 80–93% across all subgroups (hazard ratio range: 0.07–0.20).
- Remission: At 52 weeks, 60% of inebilizumab-treated patients achieved flare-free, corticosteroid-free remission, compared with 20% on placebo.
- Consistency: Benefits were observed across all categories of disease duration, baseline IgG4, and activity score.
- Safety: No new safety issues were reported; adverse events were comparable between groups.
Conclusions
Inebilizumab demonstrated consistent, meaningful benefits across all patient subgroups, effectively reducing flares and enabling many to achieve steroid-free remission. The therapy showed a favorable safety profile, positioning it as a strong potential treatment option for long-term disease management in IgG4-RD.
Key Data
- Participants: 135 (INEB: 68 | PBO: 67)
- Average disease duration: 2.6 years
- Recurrent disease: 54%
- Median IgG4 level: 653 mg/dL
- Flare risk reduction: 80–93% (HR 0.07–0.20)
- Steroid-free remission: 60% (INEB) vs 20% (PBO)
- Safety: No major differences in adverse events between groups
Authors
Yoshiya Tanaka, Emma Culver, Arezou Khosroshahi, Wen Zhang, Kazuichi Okazaki, Matthias Löhr, Nicolas Schleinitz, Xinxin Dong, Melissa Rosen, Sue Cheng, Daniel Cimbora, and John Stone.
Affiliations: University of Occupational and Environmental Health (Japan); University of Oxford (UK); Emory University (USA); Peking Union Medical College Hospital (China); Kansai Medical University (Japan); Karolinska Institutet (Sweden); Aix Marseille University (France); Amgen, Thousand Oaks, CA; and Massachusetts General Hospital/Harvard Medical School (USA).
Read the published abstract in ACR Meeting Abstracts
MITIGATE Trial Also Demonstrates Efficacy & Prevents Flares Across All Types of Organ Involvement
2020: Inebilizumab Efficacy and Safety in Patients with Common, Urgent, and Fibrotic Organ Manifestations of IgG4-RD: Subgroup Analyses from the MITIGATE Trial
Summary
This analysis from the MITIGATE Phase 3 trial evaluated how inebilizumab (INEB)—a CD19-directed B-cell–depleting therapy—performed across different organ subgroups of IgG4-related disease (IgG4-RD). Since organ involvement varies widely in urgency, severity, and fibrotic behavior, understanding organ-specific responses helps guide clinical expectations. Across all subgroups—including common organs, urgent organs (pancreas, kidney, bile ducts, aorta, orbits), and fibrotic organs (retroperitoneum, mediastinum, mesentery, thyroid, meninges)—INEB consistently reduced flare risk, lowered glucocorticoid exposure, and increased the proportion of patients achieving flare-free, steroid-free remission. Safety remained comparable across groups, with no unexpected organ-specific concerns.
Questions Answered by this Poster
- Does inebilizumab work equally well across different IgG4-RD organ phenotypes?
- Are urgent or high-risk organ manifestations (e.g., pancreas, kidney) responsive to INEB?
- Do fibrotic-dominant phenotypes still benefit from B-cell depletion?
- Does INEB reduce steroid use across organ subgroups?
- What is the organ-specific flare risk reduction compared to placebo?
Methods
- Post-hoc subgroup analysis of MITIGATE (Phase 3, randomized, placebo-controlled trial).
- Participants recently experienced an IgG4-RD flare requiring glucocorticoids.
- Randomized 1:1 to INEB or placebo, dosed Day 1, Day 15, Week 26.
- Subgroups defined by:
- Common organs (≥20% of participants).
- Urgent organs: pancreas, kidney, bile ducts, aorta/large vessels, orbits.
- Fibrotic phenotype organs: retroperitoneum, mediastinum, mesentery, thyroid, meninges.
- Outcomes evaluated:
- Risk of flare during randomized controlled period (RCP).
- Flare-free, steroid-free complete remission.
- On-study glucocorticoid use.
- Safety by subgroup.
Results
- Participants: 135 randomized (INEB n=68, placebo n=67).
- Organ involvement balanced between groups at baseline.
- Flare Risk Reduction: INEB reduced flare risk across all organ subgroups; HRs similar to overall HR (0.13).
- Largest effects:
- Pancreas: HR 0.03
- Bile ducts: HR 0.00
- Kidney: HR 0.08
- Remission: Higher rates of flare-free, steroid-free complete remission in all subgroups with adequate sample size.
- Steroid Use: INEB markedly reduced the need for on-study glucocorticoids and lowered mean daily doses across all groups.
- Fibrotic organs: Also showed clear benefit, even though fibrotic disease can be less responsive to immunotherapy.
- Safety:
- No new safety signals.
- Similar safety profile across organ subgroup categories.
Conclusions
Inebilizumab demonstrated consistent efficacy and safety across all IgG4-RD organ subgroups, including patients with urgent or fibrotic manifestations. INEB reduced flare risk, glucocorticoid exposure, and improved remission rates regardless of organ phenotype.
Key Data
- Overall flare-risk HR: ~0.13 vs placebo (consistent across organ groups).
- Urgent organs:
- Pancreatic involvement → HR 0.03
- Biliary involvement → HR 0.00
- Kidney involvement → HR 0.08
- Steroid reduction:
- Fewer patients required on-study glucocorticoids in INEB arms.
- Lower mean daily GC dose across all subgroups.
- Remission: Higher rates of flare-free, GC-free remission at Week 52 in all analyzable subgroups.
- Fibrotic organs: Still responded well despite typical treatment challenges.
Authors
Arezou Khosroshahi (Emory University, Atlanta, GA), Emma Culver (John Radcliffe Hospital, University of Oxford, United Kingdom), Wen Zhang (Peking Union Medical College Hospital, Beijing, China), Kazuichi Okazaki (Kansai Medical University Kori Hospital, Osaka, Japan), Yoshiya Tanaka (University of Occupational and Environmental Health, Kitakyushu, Japan), Matthias Lohr (Karolinska Institutet, Stockholm, Sweden), Nicolas Schleinitz (Aix Marseille University, AP-HM, Marseille, France), Xinxin Dong, Sue Cheng, and Daniel Cimbora (Amgen, Thousand Oaks, CA), and John Stone (Massachusetts General Hospital, Harvard Medical School, Concord, MA).
Read the published abstract in ACR Meeting Abstracts
European Trial Shows What IgG4-RD Looks Like in Everyday Clinical Practice
Clinical Features of IgG4-Related Disease - A Single Centre Experience
Questions Answered by this Poster
- What does IgG4-RD look like in a real-world European cohort?
- Which organ systems are most commonly affected?
- What are the typical patient demographics?
- How often do patients meet the 2019 ACR/EULAR criteria?
- What treatments are most frequently used?
Methods
- Design: Retrospective chart review
- Timeframe: January 2012 – December 2024
- Population: 58 adult patients diagnosed with IgG4-RD
- Data collected:
- Demographics
- Comorbidities
- Symptom duration
- Organ involvement
- Histopathology
- Fulfillment of 2019 ACR/EULAR criteria
- Treatment regimens
- Analysis: Descriptive statistics
Results
- 79% male, median age 63 years.
- Comorbidities: diabetes (10), asthma (10), atopy (5)
- Disease presentation:
- Median symptom duration before diagnosis: 6 months
- Average organs/regions involved: 2 (range: 1–7)
- Phenotypes: 63.8% proliferative; 36.2% fibrotic
- Phenotype categories:
- Retroperitoneal fibrosis/aortitis: 43.1%
- Mikulicz/systemic phenotype: 25.9%
- Pancreato-hepato-biliary: 17.2%
- Head/neck limited disease: 10.3%
- Unclassifiable: 2 patients
- Histopathology:
- Biopsies performed: 47
- Consistent with IgG4-RD: 36 (76.6%)
- Suspicious for IgG4-RD: 4 (8.5%)
- Classification criteria:
- 2019 ACR/EULAR criteria met by 69% (40/58)
- Treatment:
- 75.9% received systemic therapy
- Glucocorticoids: 41 patients
- Immunomodulators: 25 patients
- 3 used as standalone therapy
- 22 combined with glucocorticoids
- Surgical therapy: 9 patients
- Observation only: 5 patients
Conclusions
The Slovenian IgG4-RD cohort was predominantly composed of middle-aged men, with retroperitoneal fibrosis and aortitis as the most common phenotype. Most patients received glucocorticoid-based systemic therapy, and nearly 70% met the 2019 ACR/EULAR classification criteria.
Key Data:
- 79% male, median age 63
- Most common phenotype: retroperitoneal fibrosis/aortitis (43%)
- Proliferative phenotype: 63.8%
- ACR/EULAR 2019 criteria met: 69%
- Systemic therapy used in 76%
- Biopsy-confirmed IgG4-RD: 76.6%
Authors
Alojzija Hočevar, Aleš Grošelj, Gregor Hawlina, Matic Koželj, Andrej Skoberne, Jože Pizem, and Vesna Jurčič from University Medical Center Ljubljana and the Institute of Pathology at the University of Ljubljana, Slovenia.
Read the published abstract in ACR Meeting Abstracts
IgG4-RD Education by AiArthritis
Go With Us! To EULAR 2025: What is IgG4-Related Disease -- and Why Are So Many Patients Misdiagnosed?
In this Go With Us! video from EULAR 2025, Health Education Manager Leila P.L. Valete breaks down key insights from a session on IgG4-related disease (IgG4-RD)—a rare immune condition that’s often confused with other autoimmune diseases like lupus, Sjögren’s, or rheumatoid arthritis.
This video covers:
- What IgG4-RD is and why it’s so tricky to diagnose
- What “SACQ” means (and why high lab numbers don’t always mean symptoms)
- How doctors use histology (tissue samples) to confirm diagnosis—and where that can go wrong
- A look at current and emerging treatments like Inebilizumab (Uplizna) and Ruznilomab
- Why understanding this disease is key for “mystery patients” who still don’t have answers
Thank you to Amgen for supporting our IgG4-RD content









