Biosimilars - What patients need to know
AiArthritis Voices 360 Main, Full Episode 77
Air Date: Sep 3, 2022
This episode is Step 2 - the first time putting it on the table - as outlined in our 6 Step Patient-Led Problem Solving Process.
As more treatment options are moving towards Biosimilars, many questions are being raised by patients. What are Biosimilars? Are they safe and effective? Are there downsides? Upsides? What are doctors' opinions on Biosimilars? What should I do if I am switched to a Biosimilar?
These are all questions that are at the forefront of our minds when living with AiArthritis diseases. This month, we invited patient advocates, doctors and coalition leaders to join the conversation to discuss and answer the many questions someone living with AiArthritis may have. Join us this month as we break down Biosimilars, share opinions, concerns, research, and resources so patients can better understand Biosimilars and what their availability means for their treatment plan.
Episode Highlights :
- What are Biosimilars?
- The main concerns patients have with switching to Biosimilars
- What is Non-medical switching? Automatic Substitution? Interchangeability?
- Biosimilars are safe and effective and are recommended for patients to use if it does not interrupt continuity of care
- Regulations and the physicians involvement with switching patients to biosimilars
- First steps to take if you are asked to switch to a Biosimilar
- Research and resources to learn more about Biosimilars
Be sure to check out our top-rated show on
Feedspot!
Who is at the table?

Join Tiffany Westrich-Robertson, CEO of AiArthritis (and person living with non-radiographic axial spondylarthritis), Dr. Ralph McKibbin, Chairman of Alliance for Safe Biologic Medicines (ASBM) and gastroenterologist, Andrew Spiegel, Global Colon Cancer Association (GCCA) and ASBM Steering Committee, and Michael Reilly, Executive Director of ASBM.
This episode was sponsored by:
LISTEN TO THE FULL EPISODE THEN BE SURE TO TAKE A SEAT AT THE TABLE BY JOINING THE CONVERSATION!
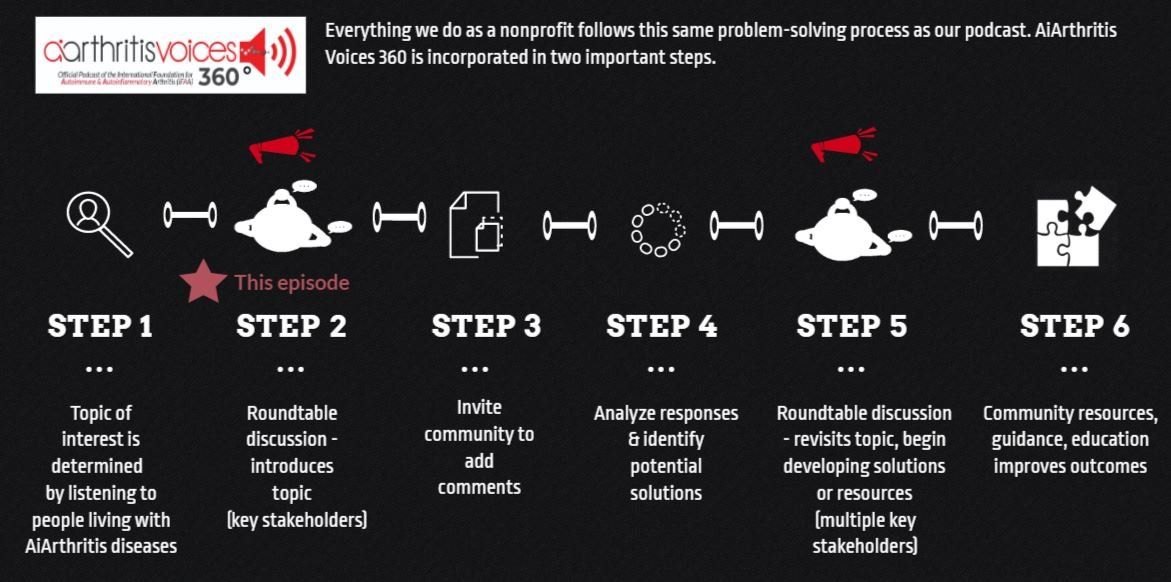
All our main 1st Sunday of the month episodes are either an initial "put the topic on the table" episode (Step 2 in our organization's 6-step problem solving process) or a "revisit to the table" episode, where we build on a past show because we have moved forward in developing help, tools, or projects around the issue (Step 5 in our organization's 6-step process).
After each show airs we spin off the conversation into many discussions over various formats, which we now call #360its (new in 2022)!
Join the Conversation - Enter Your Comments Below

And now, let's 360it!
The main Sunday episode is where we "put the topic on the table," but it's not where the conversation ends! Now we spin off the conversation into different discussion segments. Below you will find several 360its. Some are videos from the main episode, while others are audiograms (soundbites).
Soon we will be launching additional 360its, which will build on these conversations. We'll hear from patients in the United States, Canada, and Australia who are here to help you through the transition to biosimilars. We are also planning a WATCH PARTY, where we will play back segments of webinars that aim to teach you more about biosimilars - and you'll have your fellow patients at AiArthritis to talk through it all with you! Stay tuned.
SHORT VIDEO CLIPS FROM THE MAIN EPISODE
360it Short Video: What is a Biosimilar
Biosimilars are living organism and while they are not exactly identical the active ingredient is replicated from the original biologic.
360it Short Video: First Steps When Being Told You Are Switching To A Biosimilar
The prescriber should be playing a role and you should be consulting with them as a start.
360it Short Video: Transparency in Biosimilars
We need to be transparent about what these medications are and when it's safe to switch and that patients know and understand what they are taking. What are your thoughts?
Submit them using our Biosimilars Hotline.
360it Short Video: What is a Biosimilar?
Biosimilars are not identical but are tested to show there are not additional side effects, reactions, etc. They go through complex testing to show they are made the same way with similar outcomes but by a different company's process.
360it Short Video: Biosimilars vs Generic
Switching to a biosimilar is not the same as being switched to a generic medication, because biologics/biosimilars are made from living organisms and not machine made. so, no two can be 100% the same. Is it safe for you and your doctor to switch your biologic to a biosimilar?
SHORT AUDIOGRAMS FROM THE MAIN EPISODE
360it#: Physician Involvement
Physicians should be involved in the decisions for non-medical switching, not just the payer (insurance company) or government.
360it#: Data for Switching
Good data is necessary before switching to a Biosimilar, as often people have multiple factors at play.
360it#: Interchangeable Biosimilar
An Interchangeable Biosimilar is not a better Biosimilar but a Biosimilar with additional data.
360it#: Patient Resource
A webinar that is archived all about Biosimilars can be found at learnbiosimilars.org.
360it#: Best Interest of Patient
Everyone must remember the Patient whom is taking the medicines and is sick taking the medicines, and that should be the focus!
Your Co-Hosts & Guests: Who is at the table this episode?
Tiffany Westrich-Robertson
Tiffany is the CEO at International Foundation for AiArthritis and uses her professional expertise in mind-mapping and problem solving to help others, like her, who live with AiArthritis diseases work in unison to identify and solve unresolved community issues. For the last several years, she has continued her education in research, including becoming a professional focus group moderator, and translated this experience at our organization to develop award-winning, innovative projects that are taking patient engagement to next levels.
Tiffany has served on several advisory boards, including those to advance patient voices in policy, clinical trials, and precision medicine. In addition to reviewing grants at PCORI and for the Department of Defense, she was the sole patient grant reviewer for the National Institute of Arthritis and Musculoskeletal and Skin diseases from 2015-2018. She currently participates as a Patient Research Partner for OMERACT (Outcome Measures in Rheumatology), co-leads our organizations' international effort to advance patient voices in rheumatology research (the ACTion Council) and has dedicated her professional career to developing other patients to utilize their voices to impact the future of millions.
- Facebook: @TiffanyAiArthritis
- Twitter: @TiffWTobertson
- LinkedIn: @TiffanyWestrichRobertson
Ralph McKibbin, MD, FACP, FACG, AGAF
Dr. McKibbin the Chairman of Alliance for Safe Biologic Medicines. Dr. McKibbin is a practicing gastroenterologist at Blair Gastroenterology Associates in Altoona, PA. He is past president of both the Pennsylvania Society of Gastroenterology and of the Digestive Disease National Coalition (DDNC). He sits on the Member Advisory Panel of the Pennsylvania Medical Society; and is a member of the Pennsylvania State Cancer Control Consortium. Dr. McKibbin has written extensively on the issues of non-medical switching and insurance industry utilization management techniques including step therapy and copay accumulator adjustments.
Andrew Spiegel
Andrew has nearly two decades of experience in the patient advocacy arena. Spiegel co-founded the Colorectal Cancer Alliance, now the leading US based national patient advocacy organization dedicated to colon cancer. Mr. Spiegel, an attorney, besides being a co-founder of the organization and longtime board member of the Alliance became CEO in January of 2008 and he ran the CCA for nearly 5 years, before undertaking his next venture, the Global Colon Cancer Association (GCCA).In addition to his work in the colon cancer community, Spiegel is an active advocate for health care policies both in the US and now worldwide. He is a co-founder and currently serves on the steering committee of the Alliance for Safe Biologic Medicines (ASBM). He is on the Board of Directors, and in December 2014 was elected to Chair, of the Digestive Disease National Coalition (DDNC),a founding member of the Coalition to Increase Clinical Trial Participation and in May of 2016 he began a three year term as a member of the Board of Directors of the International Alliance of Patient Organizations (IAPO) where he chaired the fundraising committee. Spiegel has won multiple awards for his work in patient advocacy.
Michael Reilly
Executive Director of Alliance for Safe Biologic Medicines, has served as the executive director of ASBM since its inception in 2010. He has more than a decade of experience in the federal government developing and implementing healthcare policy. Mr. Reilly served as the associate deputy secretary at the U.S. Department of Health and Human Services (HHS) from 2005-2008 responsible for policy development and implementation, as well as regulatory oversight for issues involving CMS and the FDA. In addition to serving as the associate deputy secretary, Mr. Reilly served as a senior advisor to the assistant secretary for public affairs and the assistant secretary for planning and evaluation at HHS from 2002-2005. Mr. Reilly has been quoted in a series of FDA publications and co-authored many articles on biosimilars for the Generics and Biosimilars Initiative Journal. He has also presented to health regulators worldwide, including the Australian TGA, Health Canada and the World Health Organization (WHO).
More ways to pull up your seat at the table
What do you think about this episode?
We want to know what you think! By continuing the conversation with your opinions and perspectives - we all get a better understanding of the problems facing our community. Better yet, through these conversations we can start working and developing solutions.
We mean it when say 360. Not only do we want your input anytime and anywhere, but we also are eager to see where the conversation will take us. So please, "pull up a seat at the table" and let's start talking!
JOIN IN THE COMMENTS BOX ABOVE OR
Email us at podcast@aiarthritis.org, message us on social media (find us by searching for @IFAiArthritis)
Continue the conversation in our own AiArthritis Voices 360 Talk Show Group!
Pull up a seat and join the conversation on the topic from today and past episodes. You may even get an opportunity to talk directly with the co-hosts and any episode guests!
Love the show? Help us make sure we stay on the air by making a donation.
Your contribution helps us continue the work we do every day to improve the lives of millions worldwide.