- HOME
- ABOUT US
- WHAT IS AiARTHRITIS
- Diseases
- Rheumatoid Arthritis (RA)
- Psoriatic Arthritis (PsA)
- Systemic Lupus Erythematosus (SLE)
- Sjögren's Disease (SD)
- Axial Spondyloarthritis (AxSpA)
- Crohn's Disease
- Sarcoidosis
- Relapsing Polychondritis (RP)
- Systemic Sclerosis/Scleroderma (SSc)
- Behcet's Disease (BD)
- Palindromic Rheumatism (PR)
- VEXAS
- Antisynthetase Syndrome (ASS)
- Mixed Connective Tissue Disease
- JIA
- Familial Mediterranean Fever (FMF)
- HIDS (hyper-IgD syndrome, a mevalonate kinase deficiency)
- Cryopyrin-Associated Periodic Syndromes (CAPS) - Familial Cold Autoinflammatory Syndrome, Muckle-Wells Syndrome
- Schnitzler Syndrome
- Chronic Nonbacterial Osteomyelitis (CNO)/Chronic Recurrent Multifocal Osteomyelitis (CRMO)
- Still's Disease
- All Diseases
- Diseases
- OUR WORK
- RESOURCES & TOOLS
- GET INVOLVED
- CONTACT US
- Newly and Undiagnosed
#360it: Medicare Drug Price Negotiation: How Will it Affect Patient Access?
AiArthritis Voices 360, 360it from Episode 88
Air Date: December 27 , 2023
This episode is a 360it revisiting the topics put on the table during Episode 88 : Biosimilars - Interchangeability & Switching 2023
Join us in this breakout conversation (called a 360it) that spun off from Episode 88 Biosimilars : Interchangeability & Switching 2023. In this spin off segment, we dissect the Inflation Reduction Act (IRA) and its potential fallout on patient access to diverse treatment plans. Tracing the historical trajectory of the IRA, we uncover its role in price negotiations and the concerning shift in investments away from critical drugs tailored for specific diseases. This reallocation poses a tangible threat to the availability and diversity of treatments, including for those living with AiArthritis diseases. As we dive into the landscape of healthcare policy, the discussion emphasizes the pressing need for IRA improvements to consider the long term well-being of patients. Without these crucial enhancements, we will have a future with not only fewer innovative treatments but potentially less treatment options available.
Join us in fighting for improvements to the IRA and patient involvement in treatment access.
Episode Highlights:
- The history of Medicare Part D and its positive impact on healthcare
- What is the Inflation Reduction Act
- Why the IRA price negotiations are important to understand and how it will affect your treatment access
- How healthcare R&D will be negatively impacted by the IRA price negotiations
- Consequences to Patients of Drug Price-Setting Policies for patients
- How can patients get involved in the IRA price negotiations
- How the IRA will disincentivize small molecule drugs and indications for treatment plans
AiArthritis Voices 360 is produced by the International Foundation for Autoimmune and Autoinflammatory Arthritis. Visit us on the web at
www.aiarthritis.org/talkshow. Find us on Twitter, Instagram, TikTok, or Facebook (@IFAiArthritis) or email us (podcast@aiarthritis.org). Be sure to check out our top-rated show on
Feedspot!
Who is at the table?

Tiffany Westrich=Roberson, CEO and person living with Axial Spondyloarthritis.
Andrew Spiegel, an active advocate for health care policies both in the US and now worldwide
Michael Reilly, Executive Director of Alliance for Safe Biologic Medicines
Charles M Clapton, has nearly two decades of Capitol Hill experience,
Thomas R Barker, is one of the authors of the firm's Medicaid & the Law blog
-
Expand to View the Podcast Transcript
[00:00:00] Tiffany: Hello and welcome to AiArthritis Voices 360. This is the official talk show for the International Foundation, Autoimmune and Autoinflammatory Arthritis, which we just say AiArthritis for short. My name is Tiffany Westridge-Robertson. I'm the CEO of the organization, original founder and person living with the conditions myself, axial spondyloarthritis to be exact.
And like always on the show, I am not alone. I have people joining me here at the table and it is an exciting episode I tell you, it's a continued episode and a building on something that we put on the table on episode 88 which was about biosimilars and we talked about something called the Inflation Reduction Act or the IRA and since we put that topic on the table that gave us grounds to spin off and we say 360 or a 360it we're going to do a whole entire episode just on this 360it on the IRA and I have some amazing people here with me today that I'm going to turn over and let them introduce themselves.
First up, Michael. Hi, Michael.
[00:01:09] Michael Reilly: Hey, Tiffany, thanks for having me here today. I'm Michael Reilly. I'm the executive director of the Alliance for Safe Biologic Medicines. I have been in that role for the past 13 years and previous to that, I was the associate deputy secretary at the US Department of Health and Human Services, working alongside Tom Barker, who's also on our panel dealing with regulations on CMS and FDA and others. And we were both fortunate almost 20 years ago to be at Constitution Hall when the president signed part D, which is what we're going to be talking about today.
[00:01:48] Tiffany: That's amazing and that's really exciting introduction really just to lead into a little bit more of what we're talking about. We're gonna get a really great background in history to get everyone caught up on where we are today. So in saying that, let's move over to Thomas.
[00:02:03] Thomas Barker: Thank you, Tiffany. You know, Michael, I hadn't thought of it until you just mentioned it, but it actually is almost exactly 20 years ago.
I think it was December 8th.
[00:02:12] Michael Reilly: Oh, it was.
[00:02:14] Thomas Barker: President Bush signed the MMA. So it was exactly 20 years ago. Yeah. So hi, everyone. My name is Tom Barker. I am a partner at the law firm of Foley Hoag. I've been at this firm for 14 years. My practice focuses mainly on representing Biotech, pharma, life sciences companies with Medicare and Medicaid coverage, coding, and reimbursement issues.
As Michael said, prior to joining Foley Hoag, I was at HHS where he and I worked together. I was the general counsel of HHS represented all of the component agencies of the department, CMS, the FDA NIH, all of the various departments within HHS and, and in, in that role had an opportunity to really focus and drill down on Medicare and Medicaid and FDA regulatory matters.
[00:03:13] Tiffany: Wonderful. Tom, we're so excited to have you on one of the first time you've been on the show. So welcome, we really appreciate it. And let's say hello to Chuck. Hi Chuck.
[00:03:23] Chuck Clapton: Hi, Tiffany. Thanks for the chance to be here today. So I'm currently the head of federal government affairs for Gilead Sciences, the largest manufacturer of HIV medicines in america. I think for the purpose of this discussion, more relevantly, I had been the previously the Chief Counsel, but during the Medicare Modernization Act, the Lead Counsel at the Energy and Commerce Committee, which is one of the three congressional committees that negotiated the creation of the Part D Drug Benefit, and then spent 17 years on Capitol Hill in a variety of roles where I got to actively oversee implementation of many of the provisions of the MMA.
[00:04:01] Tiffany: Wonderful. And also, first time on the show, so we really appreciate you being here. Thank you. And last but certainly not least, hi, Andy.
[00:04:10] Andrew Spiegel: Hey, Tiffany, everybody. My name is Andrew Spiegel and my role today is as a patient advocate. I've been in the advocacy community for more than 2 decades. Most of that time in the cancer space.
I originally got involved in the cancer world in the late 1990s, having co founded the colon cancer alliance, which is the 1st colon cancer patient group in the United States. And about 10 years into that that organization, we started a new organization called the Global Colon Cancer Association that was in partnership with our European counterparts, and now I run the Global Colon Cancer Association full time as the CEO of the organization, and we are the umbrella organization to more than 80 colon cancer groups around the world. The other hat I wear is that I'm the board chair of the World Patients Alliance, and we're the largest patient organization in the world working across all diseases.
We have about 470 patient organization members from 117 countries. So, between those 2 roles, I've been pretty busy, but I never forget that Michael and I co-founded with a group of others, the Alliance for Safe Biologic Medicines more than a decade ago. So that is a 3rd hat that I've worn many, many times around the world in representing and working with Michael on safe biologic medicines and now working to ensure that the patient voice is heard with the, the IRA. So thanks again for having me.
[00:05:48] Tiffany: Yeah, thank you so much. So, that's how I actually met Michael and Andy originally was as a member of ASBM. And as I mentioned, we put this topic of the IRA on the table a few months back. We'll definitely link to that episode.
And wanted to make sure that we really took some time to break out and talk about this. As you know, we've got different patient perspectives and groups that are represented on this call AiArthritis does work with other groups around the world. We are international even though we focus on the autoimmune and autoinflammatory arthritis.
In saying that it's very relevant in the conversation today because we are going to be talking about these, this IRA negotiations. We're going to start with a history to sort of catch everybody up on where we are today. And then also the impact that it can potentially have to people living with these diseases, including access.
So what I'm going to do is I'm going to start off by just asking to give a little bit of a background on the part D. Who would like to start that off?
[00:06:58] Michael Reilly: I would recommend that Tom start that off just because he did a phenomenal job in our webinar of kind of providing that history. And I think that will launch us into a big, deeper conversation.
[00:07:09] Tiffany: Sounds good.
[00:07:10] Thomas Barker: Thanks, Michael. So, you know, I just want to say right up front, today, this is really perfect timing for this discussion because just a couple of days ago on Friday, the FDA approved the first two therapies using gene editing to cure a disease. One using CRISPR, one using virus to deliver the corrected gene into patients with sickle cell disease.
And I think it's worth asking, will the companies that are using these tools be willing to make the investment of time, resources, complex science, when they know that politicians want to impose price controls and innovative new therapy. And so I think this is really a good way to start off talking about Medicare Part D because what Medicare Part D did was to take a new approach to regulating or maybe a better word is establishing a mechanism to control pricing in a government program. Prior to the enactment of Medicare Part D, the government set prices in the Medicare and Medicaid program. There's no other way to say it. They set prices. They controlled and regulated prices.
They said this is going to be the price for a particular hospital procedure for a physician procedure, for a hospital outpatient service, for a home health service, for skilled nursing facility service and they regulated prices and the history shows of 40 years of regulating prices in the Medicare program, it didn't work. Part D came along with a different approach. Part D came along with an approach that said, rather than having the government set prices, we're going to rely on the marketplace. We're going to rely on competition to control prices in the program, and it's evident to the, it's evident to anyone that system has worked.
Part D premiums have grown far less than premiums in the fee for service Medicare program, prices for the party program grew far less than projected as opposed to today in part B of the Medicare program, and it's because the government is not setting prices. And Tiffany I'll just say one more thing, and then I'll turn it over to my fellow panelists.
And that is the inflation Reduction Act, which came along last year, the sponsors of that so called Inflation Reduction Act, use the word negotiation. Is it the federal government is really honestly going to negotiate prices? They're not going to negotiate prices. It's price setting. It's exactly the way the Medicare program has worked for 40 years.
Well, I shouldn't say worked. The way the Medicare program has operated for the last 40 years and it hasn't worked. The cost of the Medicare program has grown exponentially compared to, for example, the inflation rate in the American economy, and that didn't happen in Part D, because Part D relied on the on the private sector, on the on, on the marketplace to regulate and control drug prices.
So I'll stop there and, and see if my fellow panelists would like to comment further.
[00:10:36] Tiffany: Yeah, absolutely. I'll turn it over whoever would like to. But that was an amazing introduction. Thank you so much for that.
[00:10:42] Michael Reilly: I would just pick it up from there. I think one of the things that I know with the webinar we did back in July, which I do recommend people take a look at because Tom, Thomas went in great detail of demonstrating through data what he just articulated through words.
And it really was again, he and I working on the regulations, understanding the prospective payment system, the way the department works, realizing that there are issues every year about increases in prices, increases in costs for beneficiaries and so on and so forth. In some respects, the non interference clause was the key.
It was the key ingredient to making the MMA Part D successful. And the stripping of that, to me is, was not based on any policy reason. It ultimately became, it was a political decision. And even if you look at other elements of the IRA, the, the Part D benefit redesign, what I see is an attempt to basically sprinkle in some other things like capping out of pocket or insulin costs or premium increases even on Part D at six percent over six years as and I what I call the beginning of the trade off of innovation and access and so to me looking at the those elements that are by the way would have had bipartisan support.
The element that had no partisan, no bipartisan support is the negotiation, so called, as Thomas says, negotiation provision. And the first thing that I would say, as we are three days from the 20th anniversary of the signing of Part D, is it was a bipartisan piece of legislation. And it is generally thought in Washington that any legislation that's passed, especially in the health care realm, that you cannot get a single vote from the opposite party is probably going to be a bad piece of legislation.
And that certainly is true with regards to the negotiation provision of the IRA.
[00:12:42] Tiffany: Yeah, absolutely. And also I did attend the webinar that you're all referring to. We will make sure that we link to that as well. Medicare drug price negotiations that impact on health care development and patient access to medicines.
It was very, very good. We will definitely link to that and continue the conversation about that too. If you want to comment afterwards, I'm all ears and we'd like to continue that conversation going as well. Let's move over, Chuck, would you like to add something to this?
[00:13:13] Chuck Clapton: The only thing I'd add to what my colleagues had already mentioned is just highlighting the role of patients and the impact that the MMA had on patients. And I would argue a big part of the reason that the Part D drug benefit was so successful was patients were really put at the center. And by that, I mean, patients were given the choices of which plan that they could enroll in. They were given the choice of which plan had the best formulary that met their needs.
And because of that choice, which ultimately led to competition in the plans, I mentioned it, but premiums have remained essentially flat for almost 20 years. Which again, I would challenge any other sector of the healthcare marketplace to find any type of product where premiums have essentially remained flat for that long.
And I think as we start to talk about the IRA, what you're going to see is those both the benefit, the patient choices, but also the role of competitive plans be significantly diminished. I think that's ultimately going to be the real harm that's going to impact patients as well.
[00:14:19] Tiffany: Yeah, I totally agree.
And speaking of patients, I'm definitely going to move it over here to Andy to weigh in on that. I can tell you that as far as AiArthritis is concerned, and we started with these listening sessions that they've had also at, at CMS and two of our drugs of the 10 are on that list and extreme concern over what this is going to mean for access, what this is going to mean for innovation, how patients can be at the table, how we can get involved.
People are very worried about losing access for our conditions in particular, not all of these drugs work. So once they get to market, 40% of us will respond, 60% will not respond. So if it's working, don't try to change that, right? And that's really where a lot in our community is leading. So I wanted to tie that in into our listeners too. So they can say, well, why, why should I hear this? What, what is important to me? Why do I need to understand this and saying that Andy, I'd like to turn it over to you to weigh in.
[00:15:21] Andrew Spiegel: Yeah. Yeah. Well, thanks, Tiffany. And, and, I'll start off by saying that I'm pretty excited that ASBM is taking on this issue because I've worked with Michael and with ASBM, like I said, for more than a decade.
And Michael can tell you, he and I traveled the world along with other stakeholders in making sure that policy makers did exactly what, what you mentioned, that is put the patients at the forefront of these policy decisions. And we literally traveled, I remember traveling throughout Europe to Spain and to Italy and to Geneva, we had multiple trips to Australia to meet with the, the therapeutics good agency and with health Canada and in Latin America. And really, we were almost always the only patient and physician group or voice at the table at all of these meetings. And so the regulators listen to us, and we continue to remain involved and so the fact that is now taking on the IRA is really exciting for me because it keeps me involved and it's something that I'm pretty passionate about. Because I look at the IRA as, you know, some provisions good, but most of the provisions that we're discussing here today are not good for the patient. And ASBM has, as everybody said, this July webinar is something I'm glad you're going to post about Tiffany because everything was really a deep dive in that webinar as to why the provisions, the negotiation provisions, particularly are bad for patients.
And you know, I've been in the colon cancer world long enough to remember when there was just 1 drug for colon cancer. And metastatic colon cancer was a one year death sentence. You were lucky if you lived one year. And now we have patients who are, you know, talking about cures. We're talking about living years and years and years with this disease and making it chronic because there's now more than 30 drugs that have been approved by the FDA for colon cancer.
And that's because of the investment that was made decades ago. And what I worry about, and everybody worries about with these negotiation provisions is that, as you said, Chuck, this is not a negotiation and Thomas, it's not a negotiation. It's, um, it's the government dictating prices, and we know from where that's happening now in the world,
people have much later access to medicines. People don't get access at all to medicines that have come out in the United States, where we don't have price controls. We get somewhere between 90 to 95 percent access to new medicines within 3 months. Nowhere else in the world that has price controls doesn't even come close to having that kind of access to, to patients. I'll say one more thing and then I'll be quiet and turn it back over. And that is a little bit of a personal story because as everybody knows, the, the IRA's, small molecule penalty, which is again discussed at length at the July 26th webinar. I want to bring it up again because it's, it affects, it affects my family personally, and it affects many cancer patients personally. And that is that there's a penalty built into the IRA for, for the development of small molecules. And, I will tell you personally that 3 and a half years ago, my wife was diagnosed with stage 4 lung cancer.
And she was a never smoker so the doctors knew that there must be something wrong. She's a young person, never smoked, there must be some mutation and they were right. She has a rare mutation that caused her lung cancer to happen. And the treatment it's working for him for all these years, the treatment that's worked is a small molecule.
It was one that was recently approved by the FDA. And then another one that she's in a clinical trial for now. These small molecules are directly targeting her cancer. They're not targeting the bad cells. She's working full time. She has no active cancer in her body right now and no side effects at all from these medicines.
These are not these biologic chemotherapy like things that are, you know, killing all the good and bad cells. This is directly targeting her cancer and they work and they work really well. And I'm really sad to see that there would be something in the IRA that targets instead of incentivizing the creation of these small molecule pills.
So, that's all I have to say for this. I'll turn it back over. Okay.
[00:19:52] Tiffany: No, but thank you for sharing that story at AiArthritis that you bring up an important point. And when we're talking about advancing innovation and precision medicine, it's something that is in the early phases in AiArthritis, been in cancer for a while.
We have been knee deep and trying to push for access to make sure that the people who have the right treatments that we know that are going to work for them, that they have access to them. I have a personal fear. What is this going to do? I feel like we're going backwards instead of moving forward. And I think that you all have made points to that and saying that I did want to circle back and you mentioned a couple of the negative things that could be coming out of the IRA, what are some of the positive things or some of the good things that possibly came out of the IRA?
[00:20:46] Thomas Barker: I think the benefit redesign in Medicare Part D is probably a good thing. You know, Chuck and Michael will remember that when Part D was enacted, Congress essentially set aside $400 billion for the program and that was it.
And so in order to fit into the budgetary parameters, Congress created, with the administration's support, the so called donut hole, the coverage gap, where the benefits stop providing coverage until a beneficiary reached catastrophic coverage. And so, you know, getting rid of the donut hole and making the benefit work better for patients, that was a good thing.
But the thing is, you didn't, there was clear bipartisan support for redesigning, for creating a new benefit to Medicare Part D. Congress could have passed that years ago. It's that, it is that some members of Congress were fixated on the idea of price negotiation, which as I said is not a negotiation, will not be a negotiation at all.
They were fixated on the idea of price negotiation and wouldn't let the good parts of the, the, the IRA pass, like the benefit redesign in Medicare Part D, and that's a shame that, that it was tied to this really very unfortunate and unworkable policy, but that has the government basically price fixing, there's, there's really no other word for it.
[00:22:20] Michael Reilly: And I would add, just going back to the original design of Part D, as Thomas and Chuck both remember, and, and it's a, to me, it's a demonstration of when you can't get bipartisan support, then you're probably not passing very good legislation. In the beginning of Part D, the conversation was about whether or not the only way you could get a prescription drug benefit was through Medicare Advantage.
Because the administration and some on the Hill wanted to basically really try and promote Medicare Advantage and move away from fee for service. And ultimately, that did not bear out because they would have not probably gotten bipartisan support going in that direction. And so, to me, when I look at it again, I mentioned some of the you talk about the positive benefits, the insulin, the capping, the out of pocket, the donut hole was something that we talked about.
I, when I, when we were passing it, we had to explain the donut hole and what I used to say about the donut hole was, even having that, because once you were out through the donut hole, basically, which is the gap in coverage. It was the people that used that we're actually saving more money than anyone else, because you got 95 percent coverage once you are out of the donut hole.
And the idea was that those were people with catastrophic costs, but ultimately trying to balance out the overall cost of the program. What I'm most concerned about right now, again, as I was mentioning the idea of what the benefits, the, the new redesign to me is a signal that we're going to give you some of this, but down the road, there's going to be an impact with regards to the innovation side of this.
And there's general agreement that there is going to be an impact on the industry. Chuck could certainly talk to you about it, even from being in industry now and having been on the other side of things when the Part D was passed and ultimately it's a calculation. The biggest thing about the non interference clause was the belief that the government can do it better.
They do not really have any experience in doing it successfully, as Thomas has pointed out. And that ultimately is the biggest concern that, you know, they say, well, five years from now, we'll see how it's working. We're talking about investment that provides hope down the road. And as Andy's story demonstrates, and we all have our own, I'm sure.
Basically, the idea of, you know, hope means you have to be alive today to have hope for a month from now, a year from now. And ultimately, right now, there is, you know, there is general agreement, even by those who support the law that this will impact R&D. We just don't know how much.
[00:24:56] Thomas Barker: And, and I'll just add to that the already less than a year after the, or maybe a little bit over a year after the IRA passed, there are people who are saying that the policy in the IRA of price negotiation should apply to launch prices of drug.
I can't imagine anything more devastating to innovation than saying that the policy of price fixing would apply to launch prices of drugs. So I started off by mentioning the, the, gene editing therapies that were approved by the FDA last Friday. Companies will not make that investment that was those therapies, we are on the cusp of a revolution in medicine right now with these new therapies, and it will be halted in its tracks if Congress were to apply this policy to launch prices because it would really just stop innovation right in it as I said, right in its tracks, it would be devastating.
And I just, I can't imagine why a company would invest the time and resources in a new therapy when they knew that Congress or a government bureaucrat was going to step in and set the price of their therapies.
[00:26:11] Michael Reilly: And, and just one more thing for me that where it becomes, as we have both Andrew and Chuck here in the oncology space in general, where it becomes most harmful, especially with a small molecule penalty, is the additional indications that happen down the road, once you basically have to front load the indications in order to know that after nine years, you're going to be negotiated.
You're not going to have those additional indications that primarily are helpful in the. in the oncology space, and that's extremely frightening, frankly, for providing hope for the patient community.
[00:26:47] Chuck Clapton: The point that always resonated with me, at the end of the day, patients don't get to pick the diseases that they suffer from, nor do our scientists get to pick or choose the modality that develops the best treatments that can help treat or cure those diseases.
What the IRA does is it affirmatively picks, and I would say discriminates against, certain types of drugs over others. And both Michael and Thomas just talked about that.
I'll just pick on the small versus large molecule. For certain types of cancer, Andrew talked about this, small molecule drugs are incredibly effective in treating certain types of cancer, same for brain disease. Biologics can't cross the blood brain barrier. Only small molecule drugs are really effective for treating mental health diseases. In the world I work in, for HIV and antivirals, you need to be able to get inside the cell to be able to prevent viral replication. You can only do that with a small molecule drug.
What the IRA has done is it said that for those drugs, we're going to disincent investment going forward relative to other types of drugs. So what happens? Venture capital investors are going to move money away from investing in those types of drugs into certain other areas. Same with, as Michael mentioned, indications.
If you look at the model that has followed, has been followed successfully for the past 40 years for developing new cancer medicines, you start with third or fourth line therapies where there's no other treatment option for those patients. Basically, the no, no alternatives, no options, get approval there, and then from there you build out, you go for second, ultimately try to get the first line.
And with each of those incremental changes, you're rewarded with additional periods of exclusivity that you can keep your exclusivity of that drug on the marketplace. Then you go to other disease or other indications within cancer. Probably the best example of that I could think of is Keytruda, which has been a remarkable drug used to treat metastatic melanoma. A whole host of other cancers. If disincentives under the IRA had existed, Merck never would have pursued those other indications because they would have been subject to negotiation after a set period of time with no ability to extend that. And I think it's just now that people are starting to understand the disincentives that this is building into the system.
And frankly, I think if you look at what the Congressional Budget Office said when the IRA was passed, they got it wrong. They said that as a result of IRA, only 15 new drugs aren't going to be developed. If you look at Thomas Phillipson, who's a professor at the University of Chicago, he said the number's probably close to 139 drugs.
And then if you look at what Peter Kulczynski did, he's a venture capital investor at RA Associates. They have said that as you already see money moving away from certain types of drugs in certain disease states because the incentive isn't there. So unfortunately, I think 10 years from now, we'll be able to look back and say, yeah, absolutely.
The drugs that we were expecting to be developed aren't there. Right now, we're in a position of trying to prove the negative. That's extremely difficult. But our fear is that unless something is done to mitigate the provisions of the IRA. Ultimately, what we are going to see is there will be fewer treatments available for patients going forward.
[00:30:02] Tiffany: Yeah, that's, that's 100 percent the concern that you hear with patients and you know, I just want to preface to a lot of patients are still new to this. They're trying to wrap their brains around. What does this all mean? And I think for me, speaking to the patients who are listening to this. That's the one important takeaway that I would like people to understand.
It does take a lot of time and a lot of money to get these innovative treatments to market. And those were great examples about in cancer, in AiArthritis diseases. Ours are also treated with small molecules. Some of the diseases are rare following a similar model to what was just mentioned. Some of us not so rare in our diseases, but we tend to filter through these. And what happens when these are lifelong average age 20 to 40 and adults, any age and children, if I can't find the right treatment, or if I don't have access to something, if, and when this one fails, I could be on a biologic. So I'm in my nineties until, you know, my life is over.
There is very little chance of remission often for diseases like the ones that we have. And so we have to think about that too. What happens to whole communities if we don't have other options that that's just to me is ridiculous. We're looking at a future of decades and decades of having to stay in trial and error in developing comorbidities, including cancer and others.
So take away for those listening who are patients. Why should you get involved? Why should you understand this? Why should you join efforts with arthritis and others on this call to make sure that your voice is heard as we're going through these, that is one of the biggest takeaways right there. So I'll get off my, my rant and, and, and turn it and flip this around.
I wanted to jump ahead. I, we have an outline in front of us, but there was a couple mentions before on the consequences. We're talking about the the consequences to drug price setting policies for patients and going into sort of U. S. leading the world in innovation and looking at models that are used from other countries.
Can somebody weigh in a little bit on that on the future of this model and what we may know based on what we're seeing other places or what it might mean to us if we lose the innovation lead in the United States.
[00:32:27] Michael Reilly: Well, I can weigh in on that, but I want to just bring up one point that keeps coming back to me through the lens of the 20th anniversary of Part D, and that is when Part D was passed.
Uh, after we passed it, uh, signed into law 20 years ago, uh, it was incredibly unpopular. It was passed right before a presidential election year. It was politicized as the IRA has been in the opposite way. And Thomas knows in the department, we had to go out and and basically try and convince the Medicare population, the public at large, but mostly the Medicare population to sign up for the prescription drug benefit with a lot of negative publicity.
And so you know, essentially it was in the same way you hear about the IRA. The IRA has been, you know, it's been polled as to the concept of negotiation and is it popular to negotiate? The question really for the patient population, when we educated the party population back then, and I was fortunate to be able to meet directly with beneficiaries and the president and talk specifically about what it would mean for them.
We knew that at the end of the day, what we were saying was true. And so working with Secretary Leavitt after Secretary Thompson, we went out and moved the popularity from, you know, 20 percent in the beginning to 90% just a couple years ago, and that's because people begin to see the benefits. The flip side here is that the question for the patient population on the IRA is, okay, well now the government can negotiate, "negotiate"
what is the downside to that? What does that mean? And ultimately, you know, for example, the way quality, the quality you talk about, you know, the European system where they look at things like quality adjusted life years and tell patients that we're going to make the equation, we're going to figure out whether or not this particular drug is available to the general population that's on a government benefit.
And by the way, we're going to say that this is for the common good, because if it's not available, we're going to offer you other things. You hear this a lot in Europe. If you look at what CMS is using to determine how they negotiate drugs, comparative effectiveness has the ability to fill in QALY. While everyone distances themselves from QALY and says no, no, no, we're not going to use QALY, we can't use QALY, that's specifically written into the law.
But we can use comparative effectiveness, which is basically what the HTA uses, where you start to compare whether or not it's effective compared to another drug, whether and how, how effective it is and you take it out of the hands of the patient population, as Chuck kind of alluded to, to determine what works best for them.
And you're doing it by limiting what is available to them. And so the European system, we've all talked about what they have for access. They do not have access to new medicines at the same time as we do. And the question is, how well is that known in the general public? It's not, because the European system is barely understood here, not really even understood by those that decided to pass the IRA, and certainly not understood by the overall patient population.
[00:35:41] Tiffany: Thanks for that, Michael. Just as a little interjection for listeners for who follow AiArthritis and some of our work and our peer led education, grassroots and the health technology assessments, value assessments is one of the topics that we have. And I'm just glad that you brought up the issue with Europe and quality.
And these are things where you look at different health systems and it means something different. So just as a side note, The Health Technology International Group is having a policy, a policy convening meeting in San Diego in January of 2024. So at the time stamp of this, it's about a month away, and I am invited to be one of the four expert patients on HTA.
And in regards to speaking with people in Europe, I'm also the co lead of the new ICER patient council. So, very Very interested and we may be doing a 360it expansion on how HTA also evolves here into the IRA because there's a lot we could talk about with that as well. But thank you for Michael and bringing that up.
Others who wanted to weigh in on or build on what Michael just said.
[00:36:55] Thomas Barker: Well, I think Andrew made the point earlier, which is that the at the end of the day as the IRA is implemented over the years, it's going to result in fewer innovative therapies being available to Medicare beneficiaries, to all people in America.
It's, it's going to hinder the ability of manufacturers to want to bring new medicines to market, and it is going to reduce the availability of innovative therapies. And, in addition, it's not going to work. It's not gonna, it's not gonna lower drug prices. It's not gonna lower beneficiary premiums because the entire history of rate setting in the Medicare program has shown that it is a failure.
It is a four decade failure going back to the early 1980s. Congress created the payment system for inpatient hospital services that still is in existence. It has not a in any way, shape, or form controlled inpatient hospital spending, the government just setting a rate for a service, setting a permissible rate of growth in the program has not resulted in lower costs in the Medicare program.
Michael remembers this from the days that we were HHS, we every, every, every time that CMS would issue a payment regulation, whether it's for inpatient hospital services, outpatient, physicians, home health, a group of us would sit around in a room and make a decision. Okay, how much is the inflation rate going to be?
How much are we going to pay for this particular DRG? How much are we going to pay for this particular CPT code? Imagine the absurdity of that, that a bunch of bureaucrats are sitting around in a conference room in the sixth floor of the Humphrey Building in Washington, DC deciding how much is gonna get paid for a particular test or a procedure, or a therapy or diagnostic.
It really is just, it, it it, the, the history is replete with examples that that just does not work to lower prices and the same thing is going to happen here. They can dress it up however they want. They can call it negotiation. They can call they can use the use a much overused word setting a fair price.
It's not going to work.
[00:39:27] Michael Reilly: Yeah, and I would just, I want to just add one thing and that is going back to Chuck talking about whether it's 15, whether this CBO saying that 15 drugs won't be developed or whether it's the list of 139. The, the question is, there's an acknowledgement that we have drugs that are not going to be developed.
And so the question is, are you willing to be on that list? Do you know which ones? No one can say
[00:39:51] Thomas Barker: the list of 15 or the list of 130. Exactly. That's the point. If one of those 15 drugs is a treatment for ALS, do you want, do you wanna have ALS and be told that even if it is only 15 drugs, do you wanna be told, well, you're not gonna get this therapy because we've decided it's not worthwhile to invest in this, in the research that's necessary to bring the structure to market. Sorry to interrupt Michael, but I just
[00:40:14] Michael Reilly: Great point. That's exactly the point. And that's something that goes back to my I've talked before about when Chuck and I used to work on the hill together and patient groups would come in and talk to us about funding at NIH. And we always said, go talk to NIH.
That's why Congress basically gives them the funding and they figure out where it goes because we didn't want to deal with which patient groups would come in and make an argument on behalf of one patient group against another. And so ultimately it really does matter. We don't care whether it's 15 or 140 patient groups should understand that they could be one of the 15 if you want to use it and you're acknowledging that there is going to be a number, let's just say somewhere between those two numbers.
And who is going to be on that list, they can't say with any certainty. And so ultimately, stories like Andy's and as I said, we all, I have a mother in law who died from ALS. We can all talk about, you know, how you incentivize someone to look into these things and ultimately provide any kind of hope. And the reality is, back to Thomas's point, it's bureaucrats that are doing this, it's CMS, and basically they can't do it without the HDA community, and I could say, Tiffany, when you talk about the San Diego meeting, I was in Boston for the ISPOR meeting back in, Thomas, that's when you and I have dinner.
That meeting is an HDA meeting in which they all talked about launch price. That is where they want to go. They want to be involved in launch price. They say there's no point to HDA unless we're involved in launch price. So it's going to be coming. This is the beginning, not the end with regards to what they're going to do in terms of negotiations and expanding the list and expanding the criteria.
[00:41:51] Chuck Clapton: There's two points to follow on to the points Michael is making. If you look in the Senate, there's already the smart act that's been So called introduced, I believe it's currently 30 democratic senators are co sponsoring that. So a majority of Democrats in the Senate, they would take negotiation from a small and large molecule to five years.
That was also in president Biden's budget proposal, similar proposals in the house would significantly expand and accelerate the number of drugs that are subject to negotiation. So given the overall budget crisis and crunch that Congress will be facing the, the push to expand price controls beyond where they are today is going to happen. In the sponsors of those proposals are explicit, they want to achieve European style prices. And this goes back to the point that Tom was making and like what, what has happened in Europe as a result, what we know in the UK, the number of clinical trials for new innovative new therapies has significantly declined.
Also, overall survival for cancer is significantly worse in the U. K. and other European countries relative to the United States, largely in part because they don't have access to the newest, most innovative medicines. We know in the U. K., we can't even do clinical trials on certain cancer therapies because the standard of care is such they don't even have access to what patients, as a matter of course, are available and able to use here in the United States today. The delayed access. Across Europe, we have seen that patients can't get access to the newest, most innovative therapies. Even after they're approved by the regulatory authorities, they may be approved from a regulatory perspective, but then these same agencies, whether it's NICE in the UK, or others across Europe, simply won't pay for those medicines.
And again, the people get very upset, understandably, about the prices that are charged in Europe. Nobody talks about the trade offs that are being made in those countries that mean that patients cannot get access to the most innovative medicines that could potentially save their lives.
[00:43:52] Thomas Barker: I want to just pick up on a point that Chuck just made about the desire by some in Congress and some influencers or opinion leaders in this country to expand the list of drugs that are subject to negotiation.
I just read an editorial in the Washington Post a couple of weeks ago suggesting that the solution to the high cost of the new GLP 1 agonists that some people take for weight loss, others take for A1C control for diabetics, that the solution to the high cost was to have, um, a grand compromise where Medicare would now cover weight loss drugs In an exchange for that, the government would negotiate the price of those drugs.
I mean, I can't imagine a more ineffective and unproductive idea than setting the price for, for these new therapies. Why? Chuck's company knows this better than anything else. Chuck's company discovered a cure for hepatitis C and it had a high list price and everyone, there were cries of outrage about the, the price for Sovaldi.
And what happened after a couple of years? Competition happened. It lowered the price and that's exactly what's going to happen with GLP 1 agonists and any other drug that has a high list price. Eventually competition is going to come in and it's going to lower the price because the marketplace works and government price setting doesn't work.
And, and, and, and there's really no better example of the hepatitis C therapies as they started to be, as, as there started to be competition in that market, the price started to come down and it's a cure and it's a cure.
[00:45:45] Michael Reilly: Yeah, I mean, that's amazing.
[00:45:50] Tiffany: And just on the, the note of the out of pocket costs and the high cost, you know, we all have our opinions regarding better ways to address this and fix it.
What thoughts do any of you have on this in particular? Could be pharmacy benefit managers or, so it comes to my head.
[00:46:15] Chuck Clapton: Certainly, if you look at the amount of money that's flowing through the system is not going to manufacturers. Instead of going to entities that provide little or no value, the PBMs would seem to be a logical place if you look at the price differential between the net price in terms of what manufacturers actually get versus the list prices that we are charged, that is being driven by the PBM industry.
And that that creates significant challenges for patients because they're charging their copays and out of pocket costs off those higher list prices.
And there, there's legislation about the House and Senate that we hope is going to help at least address some of those issues by requiring that these beneficiary cost sharing must be tied to the actual prices of PBMs are negotiating for those medicines.
And I think if we could achieve that, doesn't solve all the problems, but at least would be a significant benefit for patients.
[00:47:11] Tiffany: Absolutely. And we also will link to this episode for those out there who are interested in getting involved in writing letters or about making sure that you have access. There are several of these bipartisan efforts underway to try to address the transparency needs and pharmacy medicine, but managers who are these middle people negotiating these prices, I did want to just a side note as part of participating.
I participated in the CM listening CMS listening sessions. Also, another whole could be a 360 at offspin of the prescription. the prescription drug affordability boards that are happening in all the states also with the price setting and the commonality that came up was patients were unanimously saying the affordability isn't an issue for me.
The, the problem is I skipped doses or this happens because of the pharmacy benefit managers and utilization management. So in addition to we're talking about the high cost, we, we really have to also at some point breakout and talk about what the reality of PBMs are having or talking about access and the true cost of these drugs.
So I'm glad that you wanted to talk a little bit about that. Did anyone else want to want to weigh in on out of pocket costs or thoughts?
[00:48:38] Michael Reilly: Well, I mean, all I would say is that we've all discussed the fact that, you know, there are some provisions within the IRA that what I say is could have gotten bipartisan support.
They could have passed it. This was the big target of the IRA was the political win against basically, the negotiation provision. And I think we in the period of time that we've already talked about today have made it very clear that there are going to be major downstream negative effects from that provision.
No one can quantify them either way. And the question you ultimately have to ask yourself is are you willing to be the patient group that's affected by the medicine that's no longer available to you? Andy talks about it with regards to his wife's situation, which is three additional years. I, as I, as I will just offer one personal story of my own, which is that my brother in law who died of thyroid cancer also was diagnosed when he was 24 years old.
He basically was told at that time that he would live six months, and when I used to talk with him, he said he died at 36. In the 12 years, he had five children, he went to medical school, he did a residency, he got married, etc., etc. You cannot quantify the value of those 12 years of life that he used to say.
Specifically, he was, it was staying alive from medicine to medicine to hope for something that would solve it for good. But knowing that things change in a six month period, you could be from one medicine to another medicine to another medicine. And the period of time for him was 12 years. That's a significant period of time when she lived an entire life.
And it's something Andy on over the last 13, Andy talks a lot about more weddings, more graduations, more, that's a real life example. I just came from, a funeral of a relative who died of cancer and all of his children who are now grown, you know, we're there and it's an amazing testament to the industry, what it did to keep him alive.
And he was, very aware of what it was that was allowing him to live, knowing that at the end of the day, he still passed away at 36. That's incredibly difficult, but he also was greatly appreciative of what the industry did to keep him, really to give him an entire existence and to provide five children to the world.
[00:51:03] Tiffany: Andy, did you want to add anything?
[00:51:07] Andrew Spiegel: I mean, everything has been said so, so well by the other speakers. There's so little for me to add. I would just be echoing this is a really bad thing for patients long term. We're going to see less drugs. We don't know how many less drugs, but, whatever that disease group, that's not going to get their drugs.
It shouldn't be that way. We have the technology, we have the innovation and, and the money's there. So this law, as Michael has said, is a political win, but it's a loss for patients long term. And the only thing I think I would add that I think is worthwhile for the listener is what do we do now? Where do I get more information?
What can we do about this? And we've already talked about going back and watching that July 26 recording because there's a lot of valuable information and that, but there was also a white paper that was put out by ASBM I think and a couple of authors, that was publishing Gabby, recently and people should look for that.
Michael, I don't know if you have one. It's on the microsite. Yeah. Okay. So if that's on the microsite. That was the next thing I was gonna say is that ASBM has built, a micro a website for this exact issue that lays out all of the things we've been talking about. And is it, um, is it Ira?
[00:52:32] Michael Reilly: Yep. Ira Patient info.
[00:52:34] Andrew Spiegel: IRAPatient.org or.com. Dot org. Dot org. Dot org. So Ira, Inflation Reduction Act patient info work, and it's got the sample letters. I think the statements and comments that, , has sent into CMS and all this information. So, I suggest people go to that website to learn more about this and see what we can do.
My organization has actually filed a lawsuit against the administration over the IRA provisions that we're not happy with. So for all the reasons that we have talked about today, we actually have a, a lawsuit pending against CMS and the administration on this provision. So that's one of the ways we're fighting it.
But not all patient groups can do that so what they can do is to, sign on letters and they can do op eds to newspapers and ASBM can help with all of those things. So that's kind of where I wanted to close it out is that there's some action steps that can be taken.
[00:53:36] Tiffany: Yeah, thanks for that. And I'll actually piggyback on what you were saying to so AiArthritis as a member of ASBM so you can also we will connect you to those as well. In addition, AiArthritis is going to be leading a national coalition for price setting. It will include the IRA for CMS it will also include the prescription drug affordability board. So that is underway in development and already had a first informal coalition meeting and we got to get a name.
So, but that is, we don't have a name yet. So as of as of December, but that is underway and that in one of the unique uniqueness of this coalition is in addition to patient organizations cross disease because we are all affected by this price setting is we also want to have extension of groups like ASBM, other coalitions that are doing amazing work so we can connect patients to that. And in addition, we're going to have a second level, the patient coalition. So we'll be recruiting patients to be part of learning. And so that we start to recruit people, have an education component where we're led by people teaching patients about these. And then hopefully as the opportunities to have voices, we will have education already up and running and a whole pool of patients who will be comfortable to be able to, whether that's speaking, submitting comments, joining us as we submit comments. So there's gonna be a whole lot of ways that patients can get involved. So we're really excited about That as well.
[00:55:19] Michael Reilly: Can I just say one thing to all of that too?
And first of all, I want to especially thank Thomas and Chuck who are in my in our webinar as well. My perspective on this is for people to remember that there is an education component that needs to happen nationally. I mean, we literally Thomas and I, you know, at the national level had to educate the entire Medicare population.
And that's a difficult task. And we had the opportunity of having the White House and every, you know, other entity behind us. It is a big task. Number two, the thing to remember is that this is there's multi levels to this whole overall battle on this issue. The lawsuits are one level to deal with this issue.
There's also obviously the educational level for not only the patient population, but obviously also policymakers at the state and the federal level. And then finally, much of this is going to be dealt with obviously internally at CMS and HHS. So there is an internal component that is absolutely will be reflecting the administration that could change in the next election.
So there's many, many ways for this, um, uh, continuing battle to go on and to always keep in mind ultimately that, you know, the implementation implementation phase within the department is one. No one has mentioned on this call. It's one thing we did talk about on the on the webinar. The fact that CMS has chosen to go through a very quick process, not through notice and comment through guidance, to implement quickly, which is a detrimental down the road shows lack of concern for the patient or any frankly public input that they have to consider, which is completely the opposite of what we did through Part D.
Thomas talked about the amount of time we spent on rules, putting out rules for comment in Part D and ultimately there means that there's no real requirement by the department to listen to patients at all. So it's going to be really critical that we are able to kind of articulate that both on a grassroots level, but also on a kind of inside both the hill and the department, meaning HHS, because things are going to, they're already happening.
The list is out, they're already working. And ultimately you can both try and get rid of the law, but it's. You know, you also want to be able to affect the implementation of it as well.
[00:57:47] Tiffany: Yeah, that's a great point, Michael. Okay. Well, we are getting to that point where we're wrapping it up. So, in saying that, I also wanted to ask Tom, did you have any additional comments or thoughts?
[00:58:02] Thomas Barker: The only other thing I was going to say, Michael actually just said it, which is the process that the administration is using to implement the IRA leaves very little opportunity for public comment. Andy, I believe your lawsuit addresses that issue. In fact, it is quite the opposite of what we did in Part D, which is we wanted to have a robust public comment process and we did in fact have one. And it was important for us to do that and it's a better program because of it. And I think the IRA is going to be a worse program because they didn't really solicit public comment in any meaningful way.
[00:58:37] Tiffany: And that, that will be another extension to this that it will make sure we follow up on, I know that there's a lot of organizations out there that are commenting on the process and thoughts and feedback back to CMS on the process of involving or lack thereof of patients, voices, and, even just the difficulty that was around that.
So that is a whole other again, conversation that I would love to continue to talk about more. But thank you for those for those comments, Tom and Chuck is there anything else that you would like to add?
[00:59:14] Chuck Clapton: So thank you both to you, Tiffany, you know, also for Andy, that as I look at the entire debate around drug pricing, whether it's IRA or what's happening in the States with PDABs, the key is if we're going to be able to mitigate the worst aspects of this, it's going to have to involve getting patients engaged because right now that folks on the other side who are pushing this have argued that there's no negative impact to patients.
I think based on the past hours discussion, I think it's very clear that that's not the case, but until and unless we can get the patient community engaged in their voice, telling their stories and their concerns with this, we're just going to see more of the same. We're going to see these price controls expanded.
So thank you for the opportunity to do this and thank you for all the work that both of you are doing.
[01:00:00] Tiffany: Yeah. Thank you so much.
[01:00:02] Thomas Barker: Yes. Let me echo my thanks as well. You should have said that. Sorry, but I appreciate it very much. Also.
[01:00:09] Tiffany: Well, and thank all of you so much for coming to the table and having this conversation.
There's so many amazing points that were, we are discussed and of course, like always on the show that just opens the door to continued conversation. So who knows, maybe we'll be back in a few months, continuing the conversation. And as we move forward and navigate these sort of unknown of where we're going to go here in the future.
And saying that you can find this episode on our website at AiArthritis.org/talkshow. And then also if you're interested in learning more about signing up with any of our advocacy programs including this new coalition we're talking about or any of the peer led education programs, you can find that at AiArthritis.org/advocacy.
And other than that. We always invite you to take a seat at the table and continue the conversation. So feel free to find us on any social media channel at I F A I arthritis, or go ahead and send us an email. If you would like at info@AiArthritis.org excuse me. And we will be more than happy to continue this conversation and saying that again.
Thank you to the guests today. This has been an amazing conversation. Looking forward to continuing it because I think together and only together are we going to be able to move the needle on this very important issue. So thank you all so much.
[01:01:39] Michael Reilly: Thanks everyone.
[01:01:40] Chuck Clapton: Thanks.
AiArthritis Voices 360 is produced by the International Foundation for Autoimmune and Autoinflammatory Arthritis. Visit us on the web at www.aiarthritis.org/talkshow. Find us on Twitter, Instagram, TikTok, or Facebook (@IFAiArthritis) or email us (podcast@aiarthritis.org). Be sure to check out our top-rated show on Feedspot!
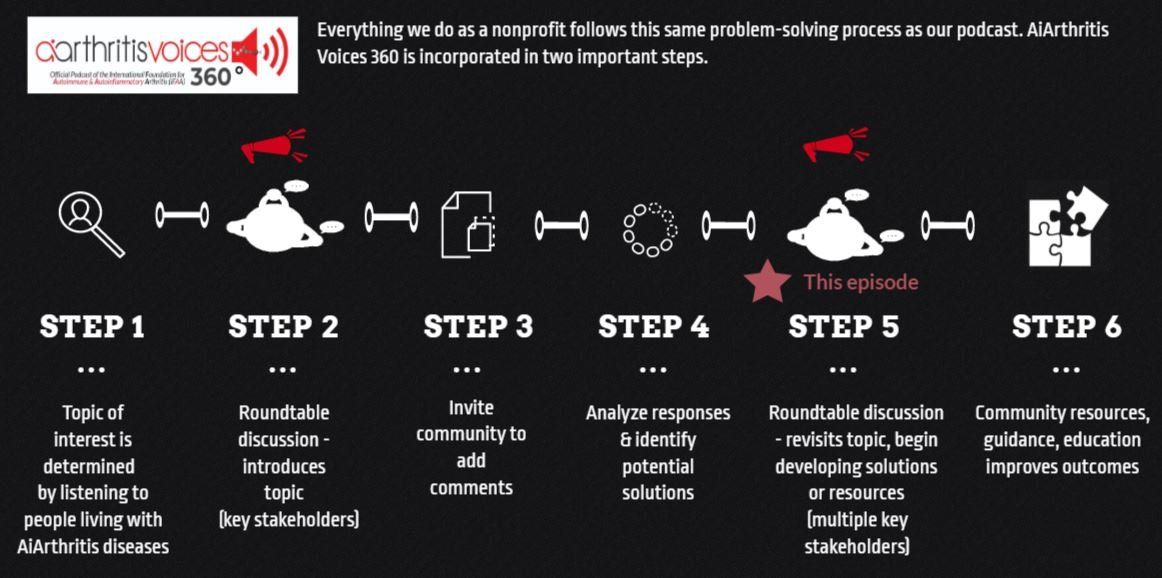
All our main 1st Sunday of the month episodes are either an initial "put the topic on the table" episode (Step 2 in our organization's 6-step problem solving process) or a "revisit to the table" episode (Step 6 in our organization's 6-step process), where we build on a past show because we have moved forward in developing help, tools, or projects around the issue (Step 5 in our organization's 6-step process).
After each show airs we spin off the conversation into many discussions over various formats, which we now call #360its (new in 2022)!
-
Additional Resources & Information
- Donate to Support the Show: https://www.aiarthritis.org/donate
- Sign up for our Monthly AiArthritis Voices 360 Talk Show newsletter! HERE
- Go with Us to Conferences HERE
- Volunteer with us! HERE
- AiArthritis Biosimilars Hotline: https://www.aiarthritis.org/biosimilars
- EMA - What Is A Biosimilar : https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview
- ASBM Statement on CMS drugs list announcement - https://safebiologics.org/medicare-price-negotiations-will-jeopardize-patient-access-to-new-medicines-result-in-worse-health-outcomes/
- ASBM webinar on IRA - https://safebiologics.org/july-26-webinar-on-ira-medicare-price-negotiations/
- An educational microsite for patients to learn more and read news articles - www.IRAPatientInfo.org
Your Host: Who is at the Table this Episode?
Tiffany Westrich-Robertson
Tiffany is the CEO at International Foundation for AiArthritis and uses her professional expertise in mind-mapping and problem solving to help others, like her, who live with AiArthritis diseases work in unison to identify and solve unresolved community issues.
Connect with Tiffany:
- Facebook: @tiffanyAiArthritis
- Twitter: @TiffWRobertson
- LinkedIn: @TiffanyWestrichRobertson
Andrew Spiegel
Andrew Spiegel
has nearly two decades of experience in the patient advocacy arena. Spiegel co-founded the Colorectal Cancer Alliance and was longtime board member of the Alliance became CEO in January of 2008 and ran the CCA for nearly 5 years, before undertaking his next venture, the Global Colon Cancer Association (GCCA).In addition to his work in the colon cancer community, Spiegel is an active advocate for health care policies both in the US and now worldwide. He is a co-founder and currently serves on the steering committee of the Alliance for Safe Biologic Medicines (ASBM). He is on the Board of Directors, and in December 2014 was elected to Chair, of the Digestive Disease National Coalition (DDNC),a founding member of the Coalition to Increase Clinical Trial Participation and in May of 2016 he began a three year term as a member of the Board of Directors of the International Alliance of Patient Organizations (IAPO) where he chaired the fundraising committee. Spiegel has won multiple awards for his work in patient advocacy.
Michael Reilly
Michael Reilly, Executive Director of Alliance for Safe Biologic Medicines, has served as the executive director of ASBM since its inception in 2010. He has more than a decade of experience in the federal government developing and implementing healthcare policy. Mr. Reilly served as the associate deputy secretary at the U.S. Department of Health and Human Services (HHS) from 2005-2008 responsible for policy development and implementation, as well as regulatory oversight for issues involving CMS and the FDA. In addition to serving as the associate deputy secretary, Mr. Reilly served as a senior advisor to the assistant secretary for public affairs and the assistant secretary for planning and evaluation at HHS from 2002-2005. Mr. Reilly has been quoted in a series of FDA publications and co-authored many articles on biosimilars for the Generics and Biosimilars Initiative Journal. He has also presented to health regulators worldwide, including the Australian TGA, Health Canada and the World Health Organization (WHO).
Charles M Clapton
Charles M Clapton. Mr Clapton has nearly two decades of Capitol Hill experience, Notably, he served as health policy director for the Senate Committee on Health, Education, Labor, and Pensions, aiding the passage of the FDA Safety and Innovation Act (2012). He also played a pivotal role as a lead Republican staffer during the Affordable Care Act's congressional deliberations. He also impacted the House Ways & Means and Energy & Commerce Committees by shaping Medicare Part D prescription drug benefits, revise drug payment methods for Medicare Part B, and Medicaid changes in the Deficit Reduction Act of 2005.
Thomas Barker
Thomas Barker
represents healthcare providers and payers before the Centers for Medicare & Medicaid Services (CMS) and other components of the Department of Health and Human Services (HHS), Congress, and the Department of Labor. Tom is a former commissioner of the Medicaid and CHIP Payment and Access Commission (MACPAC), an advisory body that provides policy advice to Congress and the states on the Medicaid and CHIP programs. He is one of the authors of the firm's Medicaid & the Law blog, www.medicaidandthelaw.com, which highlights and explains current legal and policy issues in the Medicaid program.
Pull up your seat at the table
Now it's YOUR TURN to join the conversation!
What do you think about this episode?
We want to know what you think! By continuing the conversation with your opinions and perspectives - we all get a better understanding of the problems facing our community. Better yet, through these conversations we can start working and developing solutions.
We mean it when say 360. Not only do we want your input anytime and anywhere, but we also are eager to see where the conversation will take us. So please, "pull up a seat at the table" and let's start talking!
Email us at podcast@aiarthritis.org, message us on social media (find us by searching for @IFAiArthritis)
Relevant Episodes & Projects
-
Episode #77: Biosimilars - What Patients Should Know
Learn More About this EpisodeAs more treatment options are moving towards Biosimilars, many questions are being raised by patients. What are Biosimilars? Are they safe and effective? Are there downsides? Upsides? What are doctors' opinions on Biosimilars? What should I do if I am switched to a Biosimilar?
-
Episode 88: Biosimilars Interchangeability & Switching
Learn More About this EpisodeWhat do patients need to know about potential changes that would weaken the interchangeable biosimilar designation?
-
Project: Biosimilars Education
Learn More About this ProjectWe focus on listening to comments and concerns from the patient community in regards to biosimilars. Based on that information, we help create educational materials so they understand what they are and how they may affect our healthcare system.
Love the show? Help us make sure we stay on the air by making a donation.
Your contribution helps us continue the work we do every day to improve the lives of millions worldwide.
Sign up for our newsletters
International Foundation for AiArthritis
6605 Nottingham Ave.
St. Louis, MO 63109-2661
Toll Free: 1-877-609-4226
Text: 1-314-282-7214
Copyright 2024. All rights reserved. Information on this site is intended for informational purposes only Our foundation does not engage in the practice of medicine. Please consult a physician to obtain personal healthcare and treatment options. 501(c) 3 Nonprofit Tax ID: 27-1214308.