3 Groundbreaking CAR-T Therapy Studies You Need to Know About
Early Results from a Study Testing CAR-Treg Cell Therapy for Hard-to-Treat Rheumatoid Arthritis
REGULATE-RA: A Phase 1 Study of CAR-Treg Cells Targeting Citrulinated Proteins in Refractory Rheumatoid Arthritis: Interim Report of Patient Characteristics and Safety
This poster shares early findings from the
first-in-human trial using CAR-T regulatory (CAR-Treg) cells for autoimmune disease, specifically
rheumatoid arthritis (RA). Unlike standard CAR-T cells that eliminate B cells, CAR-Treg cells are designed to
calm down the immune system and stop it from attacking the body.
For patients, this is exciting because it shows a new approach—using CAR-Tregs to
restore balance rather than destroy cells. The interim report shows the treatment was
feasible and appeared safe so far, with participants tolerating therapy and no major safety concerns reported.
Questions Answered by this Poster
- Can CAR-Treg therapy be used to treat refractory RA?
- Is CAR-Treg treatment safe and tolerable for patients?
- What makes CAR-Tregs different from standard CAR-T therapies?
Methods
- Trial type: Phase 1, first-in-human safety study.
- Participants: Patients with refractory RA (resistant to standard treatments).
- Therapy: CAR-Tregs engineered to target
citrullinated proteins, a hallmark of RA autoimmunity.
- Focus: Interim report on
patient characteristics and safety outcomes.
Results
- Patients successfully enrolled and treated with CAR-Tregs.
- Safety profile looked favorable in interim analysis.
- Early results indicate
no unexpected toxicities or major adverse events.
Conclusions
CAR-Treg therapy appears
safe and feasible for refractory RA in early testing.
Represents a
new therapeutic strategy: restoring immune tolerance rather than destroying immune cells.
More follow-up is needed to confirm long-term safety and potential clinical benefit.
Key Data
All treated patients
tolerated infusion without major safety issues.
Interim analysis suggests
encouraging safety profile.
Data still preliminary—effectiveness outcomes not yet fully available.
Authors
The poster was produced by a team affiliated with Sonoma Biotherapeutics.
Baxter SK, Bitton A, Dubovsky JA, Jensen M, Yuan V, Do J, Gordon G, Griffith M, Massarotti E, Moreland L, Sparks J, Charrow A, Pacha O, Jaleel T, Clauw A, Gabriel O, Gyurko R, Seifert J, Beilke J, Blake ML, Fox-Bosetti S, Fromhold M, Lebrec H, O'Bryan S, Pace A, van der Vuurst de Vries AR, Wang ML, Xiao Y, Arron J, Bluestone J
Initial Results of a New CD19 CAR-T Cell Therapy for Systemic Sclerosis Show Promising Safety and Effectiveness
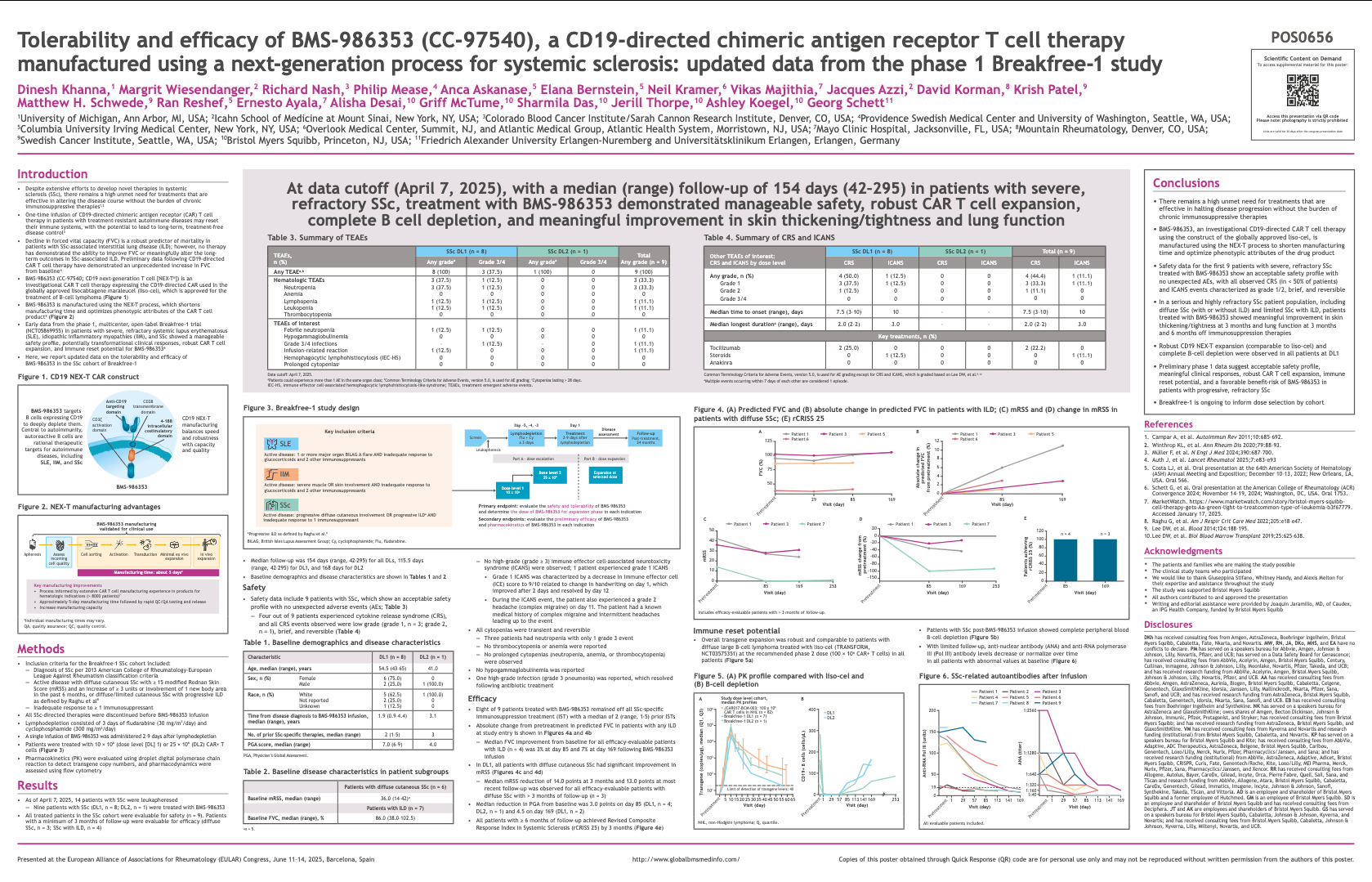
Tolerability and efficacy of BMS-986353, a CD19-directed chimeric antigen receptor T cell therapy manufactured using a next-generation process for systemic sclerosis
This poster presents early findings on
BMS-986353, a CAR-T therapy specifically developed for
systemic sclerosis, a severe autoimmune disease with limited treatment options. Using a next-generation manufacturing process, the therapy aims to be more efficient and potentially safer.
The results show that patients tolerated the therapy well, with
manageable side effects, and experienced
signs of clinical improvement in systemic sclerosis symptoms. For patients, this is important because it demonstrates that CAR-T therapy could become a viable new treatment for a disease that currently has very few effective options.
Questions Answered by this Poster
- Is BMS-986353 safe and tolerable for people with systemic sclerosis?
- How effective is CAR-T therapy in reducing symptoms of systemic sclerosis?
- What advantages does the next-generation manufacturing process provide?
- Could CAR-T therapy become a new option for systemic sclerosis patients who lack effective treatments?
Methods
- Participants: Patients with systemic sclerosis receiving BMS-986353.
- Therapy: CD19-directed CAR-T cells manufactured with a next-generation process.
- Focus: Tolerability and early efficacy.
- Measurements: Safety profile, clinical symptoms, and disease activity markers.
Results
- Safety: Treatment was
well tolerated, with side effects consistent with CAR-T therapy and manageable.
- Efficacy: Patients showed
clinical improvements, including reduced disease activity.
- Manufacturing process: Designed to enhance product quality and consistency, potentially improving safety and outcomes.
Conclusions
- BMS-986353 appears
safe and effective in systemic sclerosis patients.
- Next-generation process could represent an important step in improving CAR-T therapies for autoimmune diseases.
- Findings support continued development and larger trials.
Key Data
- Tolerability: No unexpected toxicities observed.
- Clinical response: Patients experienced improvements in systemic sclerosis symptoms.
- Process innovation: Next-generation manufacturing approach highlighted as a strength.
Authors
Developed by researchers from
Bristol Myers Squibb (BMS) in collaboration with clinical trial investigators at
University Hospital Erlangen,
Friedrich-Alexander University Erlangen-Nürnberg (FAU), and partner rheumatology centers.
Dinesh Khanna, Margrit Wiesendanger, Richard Nash, Philip Mease, Anca Askanase, Elana Bernstein, Neil Kramer, Vikas Majithia, Jacques Azzi, David Korman, Krish Patel, Matthew H. Schwede, Ran Reshef, Ernesto Ayala, Alisha Desai, Griff McTume, Sharmila Das, Jerill Thorpe, Ashley Koegel, Georg Schett
Study Looks at How a CD19 CAR-T Therapy Works in Bone Marrow of Autoimmune Disease Patients
Effect of KYV-101 Anti-CD19 CAR-T Cell Therapy in the Bone Marrow of Patients with Systemic Autoimmune Rheumatic Diseases
This study examined what happens in the bone marrow of patients with systemic autoimmune rheumatic diseases (such as lupus and related conditions) after receiving KYV-101, an anti-CD19 CAR-T therapy. The bone marrow is important because it produces the immune cells that drive autoimmune activity.
The researchers found that after CAR-T therapy, there were major changes in the bone marrow, including depletion of disease-causing B cells and early signs of immune “reset.” These findings suggest CAR-T therapy works not just in the blood but also at the source of immune cell production, which may explain why patients can enter long-lasting remission.
Questions Answered by this Poster
- How does CAR-T therapy affect the bone marrow in autoimmune patients?
- What happens to B cells and other immune cells in the marrow after treatment?
- Does bone marrow rebalancing help explain remission after CAR-T therapy?
- Can studying bone marrow responses improve understanding of long-term outcomes?
Methods
- Patients:
Individuals with systemic autoimmune rheumatic diseases receiving KYV-101 CAR-T therapy.
- Samples:
Bone marrow biopsies analyzed before and after treatment.
- Focus:
Looked at B cell presence, immune cell activity, and changes in marrow composition.
Results
- Clear reduction in CD19+ B cells in the bone marrow after CAR-T therapy.
- Signs of
immune system reset, with healthier cell populations repopulating.
- Evidence that CAR-T activity extends beyond circulation into bone marrow niches.
Conclusions
- CAR-T therapy impacts the bone marrow directly, where many disease-driving immune cells originate.
- This may be a key reason patients experience strong remission after treatment.
- Supports the idea that CAR-T has systemic and long-lasting effects in autoimmunity.
Key Data
- Marked depletion of B cells from the bone marrow following therapy.
- Evidence of
immune reconstitution (healthy cells returning).
- Bone marrow findings paralleled clinical remission improvements.
Authors
The poster was produced by a team affiliated with Charité - Universitätsmedizin Berlin, Kyverna Therapeutics, Universitätsklinikum Erlangen, and University of Erlangen-Nuremberg.
loanna Minopoulo, Artur Wilhelm, Fredrik Albach, Arnd Kleyer, Edgar Wiebe, Anja Fleischmann, Mareike Frick, Frederik Damm, Dominic Borie, Vincent Casteleyn, Robert Biesen, Thomas Dörner, Tobias Alexander, Jan Zernickel, Lukas Hinkelmann, Elpida Phithak, Norman Drzeniek, Kamran Movassaghi, Marie Luise Hütter-Krönke, Eva Schrezenmeier, Adrian Schreiber, Udo Schneider, Georg Schett, Lars Bullinger, Gerhard Krönke, Olaf Penack, David Simon
CAR-T Cell Therapy Education by AiArthritis
Episode 113: What is CAR-T Therapy?
CAR-T Therapy is one of the most talked about advances in autoimmune research today, offering new hope for people living with AiArthritis diseases such as lupus, myositis, scleroderma, and Sjögren’s. In this episode, co-hosts Leila P.L. Valete, AiArthritis Health Education Manager, and Tiffany Westrich-Robertson, CEO and Original Founder of AiArthritis, explain what CAR-T is, how it works, and why it matters.
They walk through the treatment process step by step from collecting a person’s own immune cells, to reprogramming them in a lab and reintroducing them so the immune system can reset. This episode highlights promising results from early clinical trials including patients reaching remission and stopping other medications, while also addressing safety, access, and what is still unknown.
View All CAR-T Cell Therapy Educational Videos
More About CAR-T Therapy On Our Blog
January 15, 2026
A recap of the “Go With Us!” Patient-Led Debrief highlighting key takeaways from ACR and EULAR 2025, including early diagnosis, precision medicine, advocacy, mental health, and integrative approaches to AiArthritis disease care.
September 4, 2025
Discover the latest information from EULAR 2025 about CAR-T Therapy.
April 8, 2025
Cell therapy involves using a patient’s own cells or donor cells to reset the immune system.